Municipal water systems carry a wide range of responsibilities, from maintaining stable water quality to managing seasonal shifts in source water composition. Treatment specialists frequently work with chemicals that support clarity, safety, and consistency across thousands of distribution lines. Among the substances used in these programs, sodium permanganate has become a dependable choice for addressing several recurring challenges. By the middle of the first stage of many municipal processes, sodium permanganate water treatment already begins shaping the chemical environment needed for cleaner, more manageable water.
Municipal utilities often rely on oxidizing compounds to manage dissolved metals, improve filtration performance, and support downstream disinfection. Sodium permanganate stands out due to its controlled reaction profile and its suitability for both groundwater and surface water systems. Its role stretches beyond a single step; in many plants, it influences taste, odor, clarity, and filter lifespan.
Why Municipal Facilities Turn to Sodium Permanganate
Municipal plants face pressures that range from regulatory standards to consumer expectations. Sodium permanganate helps support these requirements through reliable oxidation. Many operators appreciate its consistency across temperature variations and its ability to act within a predictable reaction window.
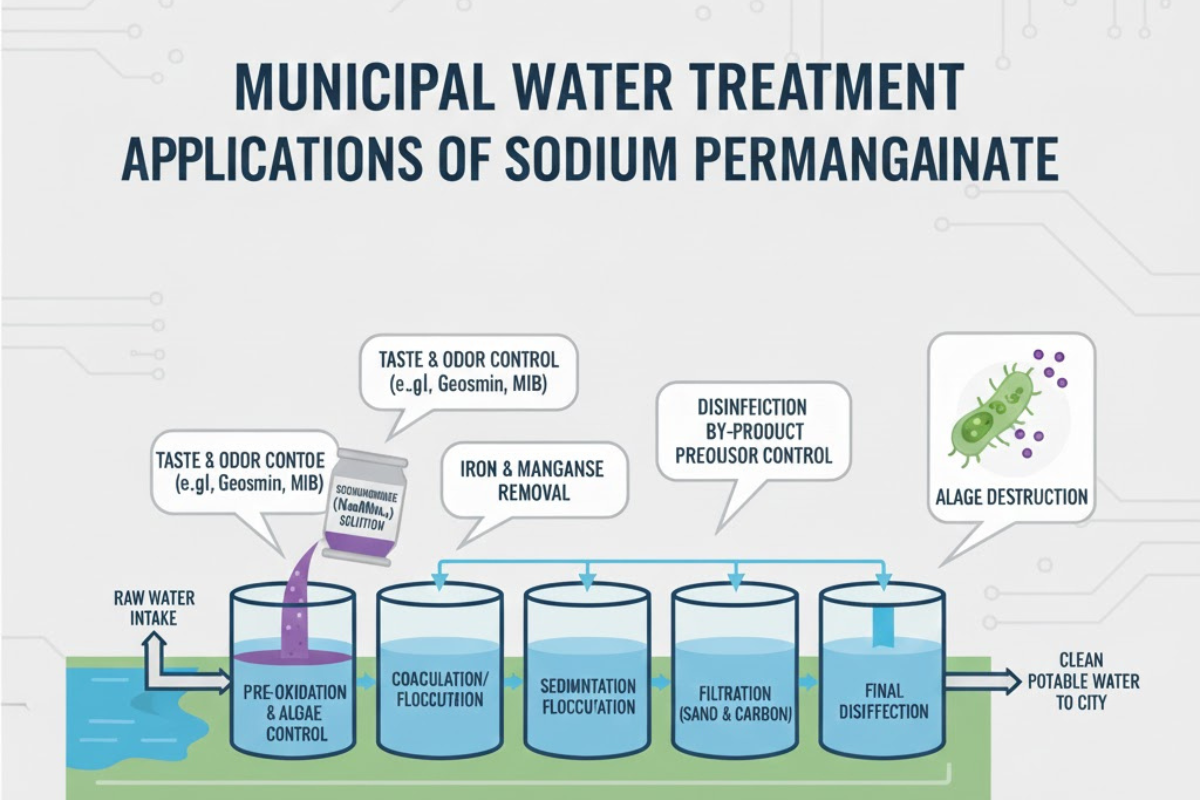
Oxidation of Iron and Manganese
Groundwater sources frequently contain iron and manganese in dissolved form. These minerals can create staining complaints, produce colored water, or overload filters. By oxidizing these metals, sodium permanganate pushes them from an invisible state into a particle form. Filtration beds capture these particles with far greater success, reducing the burden on clarifiers and downstream equipment.
Municipal systems that depend on greensand or catalytic media often use sodium permanganate as a regeneration partner. The media responds well to the oxidized metals, maintaining its activity over longer periods.
Taste and Odor Control
Some surface water sources bring challenges related to natural organic matter and seasonal blooms. Taste and odor compounds often spike during warmer months, creating noticeable changes for residents. Sodium permanganate can act early in the process, reacting with odor-producing substances and reducing their presence before final disinfection. This early-stage correction helps reinforce public confidence and provides operators with more control over quality outcomes.
Enhancing Filter Performance
Filters work best when the incoming water presents particles that are easier to capture. Sodium permanganate assists by creating more stable oxidized solids, reducing pressure swings on filter beds and lowering the risk of early media exhaustion. Plants that experience varying raw water conditions often incorporate sodium permanganate to stabilize the filtration stage, allowing more predictable backwash cycles.
How Sodium Permanganate Supports Pre-Treatment and Clarification
Pre-treatment stages determine how smoothly later processes run. When sodium permanganate is introduced near the beginning of the treatment train, it sets the stage for improved coagulation and sedimentation.
Interaction With Natural Organic Matter
Organic matter in surface water can interfere with coagulation, affect color, and react with disinfectants. Sodium permanganate helps change the chemistry of some organic molecules, making them easier to separate. By reducing certain interfering compounds early, the plant often benefits from more stable clarifier performance.
Reducing Filter Loading
By converting problematic substances into more manageable forms, the chemical helps limit the volume of material that reaches the filters in an unstable state. Less fluctuating loading extends filter life and lowers the need for frequent intervention from operators.
Applications in Distribution System Management
While most oxidation occurs in the treatment plant, sodium permanganate can also play a role in improving the condition of distribution lines.
Controlling Iron and Manganese Deposits
Older pipelines frequently collect mineral deposits from decades of variable water conditions. When these deposits release suddenly, colored water complaints rise. Some municipal programs introduce controlled oxidation to address accumulated deposits gradually, lowering the chance of unexpected discoloration events.
Supporting Stable Water Aesthetics
Residents often judge water quality based on clarity, taste, and odor long before they consider analytical measurements. Sodium permanganate helps maintain these qualities by reducing substances that cause variations in appearance and smell, especially during seasonal changes.
Safety and Handling Considerations for Municipal Operators
Sodium permanganate is a strong oxidizing agent, so careful handling remains essential. Operators often work with it in liquid form, which helps minimize airborne exposure. Storage areas typically include containment features, stable temperatures, and limited sunlight.
Over-feeding the chemical can lead to a pink or purple tint in the finished water, an indication that unreacted oxidant remains in the system. Trained technicians prevent this through accurate dosing, monitoring, and routine calibration of feed equipment. Maintaining exact measurements supports both safety and water quality standards.
Integration With Existing Municipal Infrastructure
One reason municipal facilities frequently adopt sodium permanganate is its compatibility with long-standing treatment designs. Many treatment trains already include oxidation steps, so integrating the compound rarely requires major changes.
Compatibility With Common Water Treatment Media
Greensand, catalytic carbon, and other filtration media respond well to the oxidized forms created by sodium permanganate. This compatibility eliminates the need for major renovations while offering consistent performance.
Versatility in Raw Water Conditions
Municipal sources differ widely—some rely on wells, while others depend on rivers, lakes, or reservoirs. Sodium permanganate functions across this range, giving utilities a chemical that adapts well to shifting conditions. Seasonal storms, runoff events, and drought cycles can complicate treatment; the compound adds predictability to these variations.
Value for Long-Term Water Quality Planning
Municipal operations often plan decades into the future, examining cost, reliability, and regulatory trends. Sodium permanganate supports these long-term goals by contributing stability to several parts of the treatment process. Its consistent chemistry means fewer unexpected variations, and its compatibility with widely used systems helps limit large-scale facility changes.
Utilities seeking ways to maintain clear, dependable water often incorporate sodium permanganate because its reaction patterns are familiar, tested, and supported by decades of field experience. This combination helps reduce operational challenges while reinforcing service quality.
Conclusion
Sodium permanganate plays a meaningful role in municipal water treatment, supporting oxidation, filtration performance, taste and odor management, and mineral control. Its predictable behavior, suitability for diverse water sources, and compatibility with widely used filtration media make it a valued component in treatment programs. Municipal facilities continue to rely on it as part of their effort to provide communities with dependable, clear, and appealing water throughout the year.